Abstract
Background: Asparaginases are amidohydrolases that catalyze the conversion of asparagine (Asn) and glutamine (Gln) to aspartate (Asp) and glutamate (Glu), respectively, and can therefore be used therapeutically for cancer, particularly acute lymphoblastic (and myeloid) leukemia, to deplete circulating Asn and Gln from plasma. Clinically available asparaginases are derived from two bacterial sources: Escherichia coli and Erwinia chrysanthemi (also called crisantaspase), with crisantaspases demonstrating higher glutaminase activity. In order to identify any potential contributory pathways or resistance mechanisms to asparaginases, we sought to investigate the impact of short- and long-acting crisantaspases on circulating levels of plasma amino acids (AAs) other than Gln and Asn.
Methods: Free AA concentrations were measured via a dedicated high-pressure liquid chromatography AAanalyzer (Biochrom 20 PlusAmino or Biochrom 30 Acid Analyzer). Free AAs were separated with a cation-exchange resin post precipitation of protein with sulfosalicylic acid. The AAs were differentially eluted after increasing the pH and ionic strength of the buffer. The concentration of each AA was measured by reaction of the AA with ninhydrin and detection via a colorimeter. Standard curves were generated for each AA and AAs were quantified and reported as μmol/L (µM) of AAs in plasma. For mouse studies, plasma was isolated from whole blood and delivered on ice to the Biochemical Genetics lab (University of Maryland Baltimore). For human clinical samples, 5-8 mL blood in a sodium or lithium heparin was obtained, kept on ice, and within 40 min of being collected, the plasma was separated by centrifugation (1100 g × 5 min at 4°C) and frozen at -20°C until analysis. Mouse plasma samples were from studies that included treatment with pegcrisantaspase (PegC), a long-acting Erwinia-derived asparaginase, in both immunodeficient and immunocompetent mice. Human plasma samples were from a clinical study (NCT02283190) in patients with acute myeloid leukemia (AML) treated with the short-acting crisantaspase, Erwinaze.
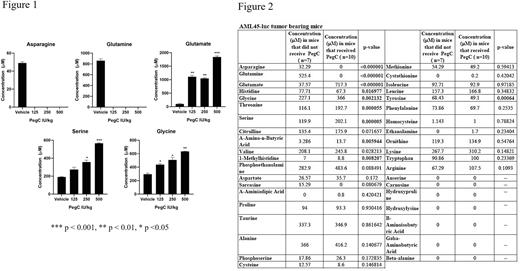
Results: As expected, in our in vivo mouse experiments, intravenous pegcrisantaspase significantly decreased plasma Gln and Asn and increased plasma Glu. Interestingly, we observed that post-pegcrisantaspase administration, plasma serine (Ser) and glycine (Gly) increased in a dose-dependent manner. In immunocompromised mice treated with one dose of pegcrisantaspase (125, 250, and 500 IU/kg), three days post-administration, Ser increased from 190 ± 6 µM in the vehicle-treated group to 274 ± 35 µM (p= 0.07) in the 125 IU/kg group, 357 ± 29 µM (p=0.01) in the 250 IU/kg group, and 565 ± 8 µM (p=0.0004) in the 500 IU/kg group. Gly also significantly increased from 295 ± 25 µM in the vehicle-treated group to 438 ± 24 µM (p=0.02) in the 125 IU/kg group, 507 ± 50 µM (p=0.03) in the 250 IU/kg group, and 633 ± 8 µM (p=0.003) in the 500 IU/kg group (Fig 1). Pegcrisantaspase also increased plasma Ser from 143 ± 7.9 µM to 301 ± 73.7 µM (p=0.02) and Gly from 251 ± 45.9 µM to 381 ± 44.4 µM (p=0.02) in immunocompetent C57BL/6 mice. In addition to non-tumor/non-leukemia bearing immunocompetent and immunocompromised mice, this phenomenon was observed in a patient-derived xenograft model of AML (comprehensive plasma AA analysis, Fig 2). Other amino acids significantly increased by pegcrisantaspase in mice include threonine and α-amino-n-butyric acid. In a comprehensive plasma amino acid evaluation in samples from AML patients treated with Erwinaze (NCT02283190), we observed a similar pattern of increase in Ser, from 82 ± 11.2 µM at baseline to 128 ± 8.3 µM on Day 8 (p=0.0007), and Gly, from 191 ± 25.8 µM at baseline to 282 ± 46.6 µM on Day 8 (p=0.006), after 1 dose of Erwinaze (25,000 IU/m2). Systematic literature review of clinical studies with asparaginases revealed that only levels of Gln/Glu and Asn/Asp have been published post-asparaginase administration; demonstrating that this report is the first comprehensive analysis of plasma amino acid levels after asparaginase exposure.
Conclusions: We report a comprehensive evaluation of plasma AA alterations in both mice and humans treated with crisantaspase. We observed a reproducible crisantaspase-induced increase in plasma Ser and Gly, which we are currently exploiting for synergism with other AML treatment modalities.
Disclosures
Emadi:Genentech: Membership on an entity's Board of Directors or advisory committees; Secura Bio: Consultancy; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; NewLink Genetics: Research Funding; Jazz Pharmaceuticals: Research Funding; KinaRx, Inc.: Other: Founder and Scientific Advisor.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal